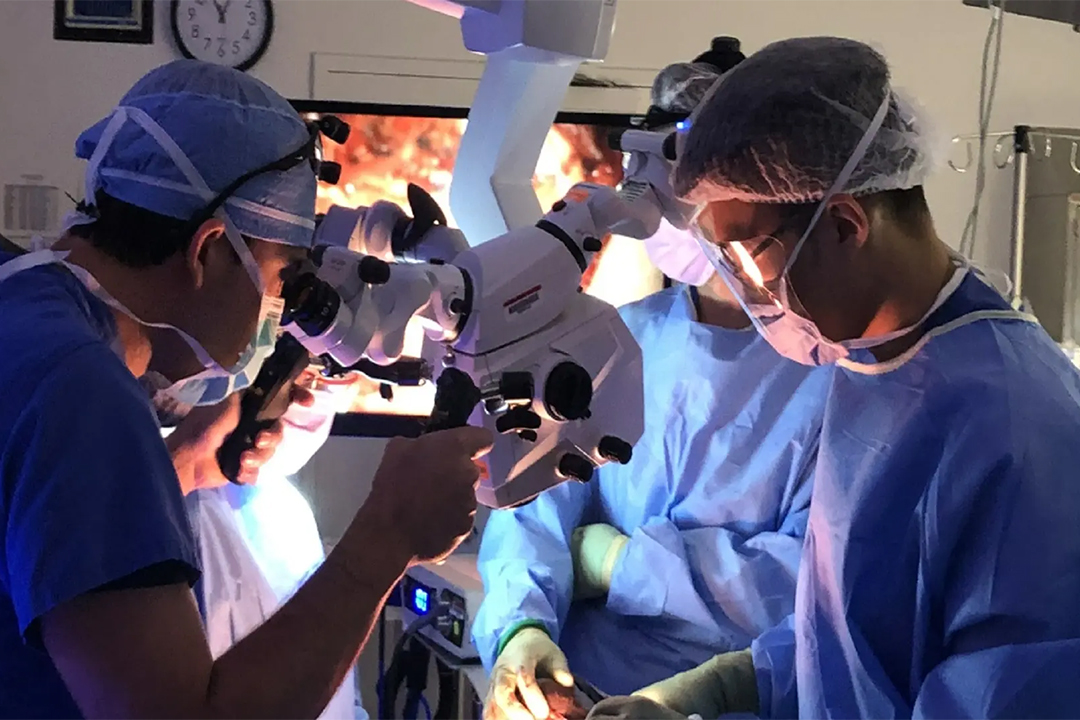
Trace Biosciences, Inc., a clinical-stage biotechnology company focused on nerve-targeted imaging, announced that the U.S. Food and Drug Administration has approved its Investigational New Drug (IND) application for LGW16-03, the company’s first nerve-specific fluorescent imaging agent. This clearance allows Trace to begin first-in-human studies to assess the safety and performance of LGW16-03 during surgery.
Helping Surgeons See What Was Once Invisible
LGW16-03, also called NerveTrace Dx, is designed to bind selectively to peripheral nerves and emit a near-infrared fluorescent signal. This makes critical nerves visible during surgery, even when they are hidden beneath tissue. Accidental nerve injuries are a major cause of surgical complications across procedures such as prostatectomy, orthopedic surgery, colorectal surgery, and head and neck operations. These injuries can lead to chronic pain, numbness, incontinence, sexual dysfunction, or voice loss, significantly affecting patients’ quality of life.
Why This Matters
“This IND clearance is a major milestone for Trace and validates over a decade of research aimed at making nerves visible to surgeons,” said Connor Barth, Ph.D., Co-Founder and CEO of Trace Biosciences. “Surgeons currently operate with limited tools to see nerves in real time. We believe LGW16-03 could significantly improve surgical safety and patient outcomes.”
Next Steps
Trace plans to begin Phase I clinical trials later this year, first focusing on safety and feasibility in orthopedic surgeries. The company intends to expand studies into other high-risk surgeries if early results are positive.
“Nerve injuries often occur because surgeons rely on anatomical knowledge and estimation rather than direct visualization,” said Nirmish Singla, MD, MSCS, FACS, urologic surgeon at Johns Hopkins Medicine and clinical advisor to Trace. “A real-time imaging agent like LGW16-03 could reduce avoidable injuries and change how these surgeries are performed.”
Broader Platform Potential
LGW16-03 represents the first step in Trace’s broader nerve-targeted platform, which could also support applications in fluorescence-guided surgery, nerve repair, nerve stimulation, and diagnostic imaging.
About Trace Biosciences
Trace Biosciences is a clinical-stage biotechnology company developing nerve-targeted imaging technologies for surgery. The company creates small-molecule imaging agents to make nerves visible and measurable in clinical settings, aiming to make surgical procedures safer and more precise.
About LGW16-03 (NerveTrace Dx)
LGW16-03 is the lead investigational imaging agent from Trace Biosciences. It highlights peripheral nerves using near-infrared fluorescence and integrates with existing surgical imaging systems to provide real-time guidance in the operating room. The agent is being studied under an FDA-cleared IND application.
Forward-looking information is subject to risks and uncertainties. Read full DISCLAIMER.